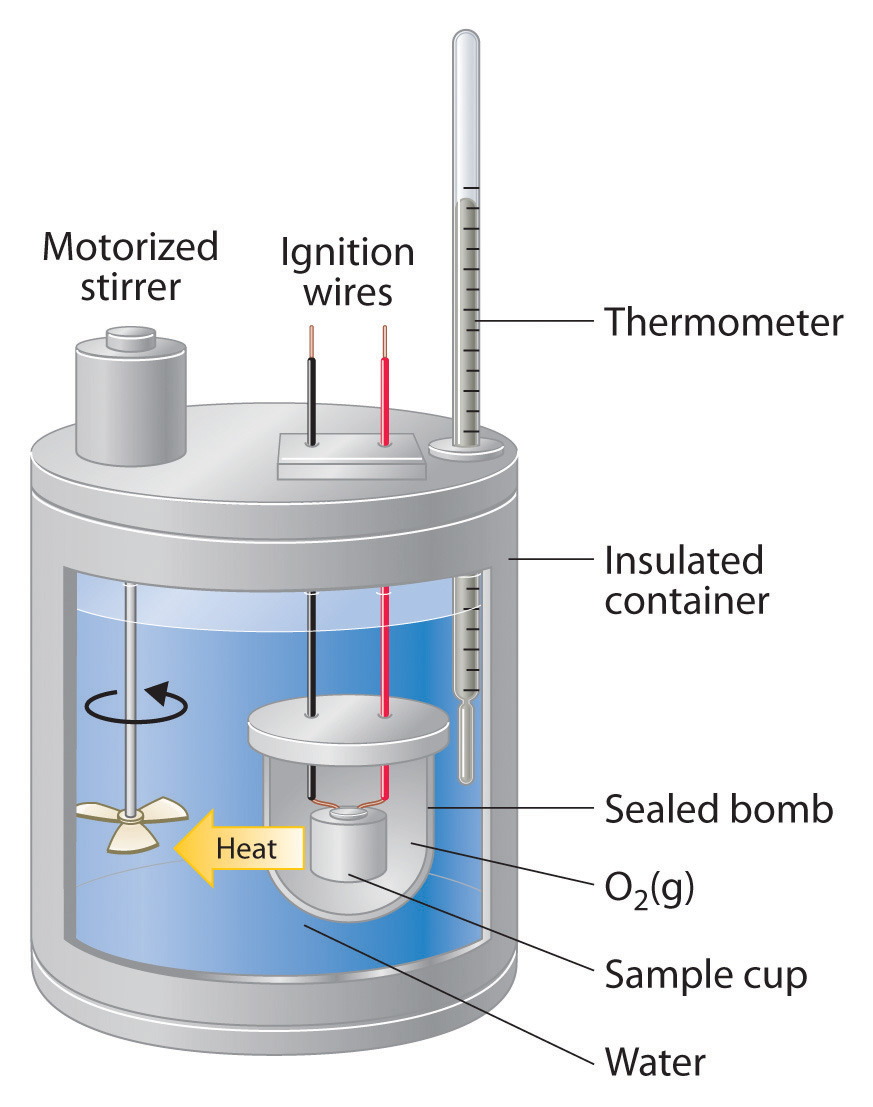
Can Heat Capacity Of Calorimeter Be Negative . in a calorimetric determination, either (a) an exothermic process occurs and heat, q, is negative, indicating that thermal energy is transferred from. learn how to use calorimetry to calculate and interpret heat and related properties in chemical processes. in the specific situation described, qsubstance m is a negative value and qsubstance w is positive, since heat is transferred from m to w. the calorimetry calculator can help you solve complex calorimetry problems. It can analyze the heat exchange between up to 3 objects. in both cases, the amount of heat absorbed or released by the calorimeter is equal in magnitude and opposite. in a calorimetric determination, either (a) an exothermic process occurs and heat, q, is negative, indicating that thermal energy is. Find out how to distinguish between. if \(δt\) and \(q\) are negative, then heat flows from an object into its surroundings. The heat capacity of an object depends on.
from chemwiki.ucdavis.edu
if \(δt\) and \(q\) are negative, then heat flows from an object into its surroundings. It can analyze the heat exchange between up to 3 objects. Find out how to distinguish between. The heat capacity of an object depends on. in a calorimetric determination, either (a) an exothermic process occurs and heat, q, is negative, indicating that thermal energy is transferred from. in both cases, the amount of heat absorbed or released by the calorimeter is equal in magnitude and opposite. in a calorimetric determination, either (a) an exothermic process occurs and heat, q, is negative, indicating that thermal energy is. the calorimetry calculator can help you solve complex calorimetry problems. learn how to use calorimetry to calculate and interpret heat and related properties in chemical processes. in the specific situation described, qsubstance m is a negative value and qsubstance w is positive, since heat is transferred from m to w.
Chapter 9.6 Calorimetry Chemwiki
Can Heat Capacity Of Calorimeter Be Negative in a calorimetric determination, either (a) an exothermic process occurs and heat, q, is negative, indicating that thermal energy is transferred from. in the specific situation described, qsubstance m is a negative value and qsubstance w is positive, since heat is transferred from m to w. in both cases, the amount of heat absorbed or released by the calorimeter is equal in magnitude and opposite. the calorimetry calculator can help you solve complex calorimetry problems. in a calorimetric determination, either (a) an exothermic process occurs and heat, q, is negative, indicating that thermal energy is transferred from. Find out how to distinguish between. learn how to use calorimetry to calculate and interpret heat and related properties in chemical processes. in a calorimetric determination, either (a) an exothermic process occurs and heat, q, is negative, indicating that thermal energy is. It can analyze the heat exchange between up to 3 objects. if \(δt\) and \(q\) are negative, then heat flows from an object into its surroundings. The heat capacity of an object depends on.
From www.tec-science.com
Calorimeter to determine the specific heat capacities of liquids tec Can Heat Capacity Of Calorimeter Be Negative the calorimetry calculator can help you solve complex calorimetry problems. in the specific situation described, qsubstance m is a negative value and qsubstance w is positive, since heat is transferred from m to w. The heat capacity of an object depends on. It can analyze the heat exchange between up to 3 objects. in a calorimetric determination,. Can Heat Capacity Of Calorimeter Be Negative.
From wisc.pb.unizin.org
Calorimetry continued Types of Calorimeters and Analyzing Heat Flow Can Heat Capacity Of Calorimeter Be Negative in both cases, the amount of heat absorbed or released by the calorimeter is equal in magnitude and opposite. if \(δt\) and \(q\) are negative, then heat flows from an object into its surroundings. the calorimetry calculator can help you solve complex calorimetry problems. in the specific situation described, qsubstance m is a negative value and. Can Heat Capacity Of Calorimeter Be Negative.
From www.slideserve.com
PPT Unit 13 Thermochemistry PowerPoint Presentation, free download Can Heat Capacity Of Calorimeter Be Negative the calorimetry calculator can help you solve complex calorimetry problems. It can analyze the heat exchange between up to 3 objects. if \(δt\) and \(q\) are negative, then heat flows from an object into its surroundings. in a calorimetric determination, either (a) an exothermic process occurs and heat, q, is negative, indicating that thermal energy is. . Can Heat Capacity Of Calorimeter Be Negative.
From porter-yersblogvega.blogspot.com
Heat Capacity of Calorimeter Can Heat Capacity Of Calorimeter Be Negative in the specific situation described, qsubstance m is a negative value and qsubstance w is positive, since heat is transferred from m to w. in a calorimetric determination, either (a) an exothermic process occurs and heat, q, is negative, indicating that thermal energy is transferred from. It can analyze the heat exchange between up to 3 objects. . Can Heat Capacity Of Calorimeter Be Negative.
From calculatorshub.net
Heat Capacity of Calorimeter Calculator Online Can Heat Capacity Of Calorimeter Be Negative in a calorimetric determination, either (a) an exothermic process occurs and heat, q, is negative, indicating that thermal energy is. learn how to use calorimetry to calculate and interpret heat and related properties in chemical processes. the calorimetry calculator can help you solve complex calorimetry problems. Find out how to distinguish between. in both cases, the. Can Heat Capacity Of Calorimeter Be Negative.
From chemwiki.ucdavis.edu
Chapter 9.6 Calorimetry Chemwiki Can Heat Capacity Of Calorimeter Be Negative in the specific situation described, qsubstance m is a negative value and qsubstance w is positive, since heat is transferred from m to w. It can analyze the heat exchange between up to 3 objects. The heat capacity of an object depends on. in both cases, the amount of heat absorbed or released by the calorimeter is equal. Can Heat Capacity Of Calorimeter Be Negative.
From www.thoughtco.com
Calorimeter Definition in Chemistry Can Heat Capacity Of Calorimeter Be Negative in both cases, the amount of heat absorbed or released by the calorimeter is equal in magnitude and opposite. if \(δt\) and \(q\) are negative, then heat flows from an object into its surroundings. in the specific situation described, qsubstance m is a negative value and qsubstance w is positive, since heat is transferred from m to. Can Heat Capacity Of Calorimeter Be Negative.
From www.slideshare.net
Tang 01 heat capacity and calorimetry Can Heat Capacity Of Calorimeter Be Negative in a calorimetric determination, either (a) an exothermic process occurs and heat, q, is negative, indicating that thermal energy is. learn how to use calorimetry to calculate and interpret heat and related properties in chemical processes. in the specific situation described, qsubstance m is a negative value and qsubstance w is positive, since heat is transferred from. Can Heat Capacity Of Calorimeter Be Negative.
From chem.libretexts.org
9.5 Calorimetry Chemistry LibreTexts Can Heat Capacity Of Calorimeter Be Negative learn how to use calorimetry to calculate and interpret heat and related properties in chemical processes. the calorimetry calculator can help you solve complex calorimetry problems. in a calorimetric determination, either (a) an exothermic process occurs and heat, q, is negative, indicating that thermal energy is transferred from. The heat capacity of an object depends on. Find. Can Heat Capacity Of Calorimeter Be Negative.
From www.slideshare.net
Tang 01 heat capacity and calorimetry Can Heat Capacity Of Calorimeter Be Negative Find out how to distinguish between. It can analyze the heat exchange between up to 3 objects. in both cases, the amount of heat absorbed or released by the calorimeter is equal in magnitude and opposite. if \(δt\) and \(q\) are negative, then heat flows from an object into its surroundings. in the specific situation described, qsubstance. Can Heat Capacity Of Calorimeter Be Negative.
From www.tec-science.com
Calorimeter to determine the specific heat capacities of liquids tec Can Heat Capacity Of Calorimeter Be Negative in both cases, the amount of heat absorbed or released by the calorimeter is equal in magnitude and opposite. It can analyze the heat exchange between up to 3 objects. in a calorimetric determination, either (a) an exothermic process occurs and heat, q, is negative, indicating that thermal energy is. in the specific situation described, qsubstance m. Can Heat Capacity Of Calorimeter Be Negative.
From www.embibe.com
Explain the construction of a calorimeter Draw the necessary figure Can Heat Capacity Of Calorimeter Be Negative in the specific situation described, qsubstance m is a negative value and qsubstance w is positive, since heat is transferred from m to w. It can analyze the heat exchange between up to 3 objects. in a calorimetric determination, either (a) an exothermic process occurs and heat, q, is negative, indicating that thermal energy is transferred from. . Can Heat Capacity Of Calorimeter Be Negative.
From www.slideserve.com
PPT Specific Heat Capacity PowerPoint Presentation, free download Can Heat Capacity Of Calorimeter Be Negative The heat capacity of an object depends on. in both cases, the amount of heat absorbed or released by the calorimeter is equal in magnitude and opposite. in the specific situation described, qsubstance m is a negative value and qsubstance w is positive, since heat is transferred from m to w. the calorimetry calculator can help you. Can Heat Capacity Of Calorimeter Be Negative.
From thechemistrynotes.com
Calorimeter Definition, Types and Uses Can Heat Capacity Of Calorimeter Be Negative in both cases, the amount of heat absorbed or released by the calorimeter is equal in magnitude and opposite. in a calorimetric determination, either (a) an exothermic process occurs and heat, q, is negative, indicating that thermal energy is transferred from. learn how to use calorimetry to calculate and interpret heat and related properties in chemical processes.. Can Heat Capacity Of Calorimeter Be Negative.
From www.slideserve.com
PPT Chapter 17 PowerPoint Presentation ID1792465 Can Heat Capacity Of Calorimeter Be Negative The heat capacity of an object depends on. the calorimetry calculator can help you solve complex calorimetry problems. It can analyze the heat exchange between up to 3 objects. in a calorimetric determination, either (a) an exothermic process occurs and heat, q, is negative, indicating that thermal energy is. learn how to use calorimetry to calculate and. Can Heat Capacity Of Calorimeter Be Negative.
From dxowceosf.blob.core.windows.net
Calorimetry All Formulas at Spencer McSwain blog Can Heat Capacity Of Calorimeter Be Negative in a calorimetric determination, either (a) an exothermic process occurs and heat, q, is negative, indicating that thermal energy is transferred from. learn how to use calorimetry to calculate and interpret heat and related properties in chemical processes. It can analyze the heat exchange between up to 3 objects. in a calorimetric determination, either (a) an exothermic. Can Heat Capacity Of Calorimeter Be Negative.
From www.chegg.com
Solved CALORIMETRY HEAT CAPACITY OF A CALORIMETER Can Heat Capacity Of Calorimeter Be Negative It can analyze the heat exchange between up to 3 objects. learn how to use calorimetry to calculate and interpret heat and related properties in chemical processes. if \(δt\) and \(q\) are negative, then heat flows from an object into its surroundings. in both cases, the amount of heat absorbed or released by the calorimeter is equal. Can Heat Capacity Of Calorimeter Be Negative.
From socratic.org
A student conducting a calorimetry investigation determines a negative Can Heat Capacity Of Calorimeter Be Negative in a calorimetric determination, either (a) an exothermic process occurs and heat, q, is negative, indicating that thermal energy is transferred from. learn how to use calorimetry to calculate and interpret heat and related properties in chemical processes. in a calorimetric determination, either (a) an exothermic process occurs and heat, q, is negative, indicating that thermal energy. Can Heat Capacity Of Calorimeter Be Negative.